
INDIA
Control Drug Standard Central Organisation (CDSCO) is India’s regulatory body regulating medical devices in the Indian market. Whether one wants to manufacture or import, both need a license to sell and distribute their medical devices. Unlike Europe, India too follows the Medical Device Regulation 2017. Apart from MDR Indian regulatory body also follow the Drug and Cosmetic Act 1940 and Rules 1945 to maintain the safety and effectiveness of the devices being marketed.
CDSCO defines medical devices as the 1
In India, at present, only notified medical devices are regulated as Drugs under the Drugs and Cosmetics Act 1940 and Rules made thereunder in 1945

1. substances used for in vitro diagnosis and surgical dressings, surgical bandages, surgical staples, surgical sutures, ligatures, blood, and blood component collection bag with or without anticoagulant covered under sub-clause (i);
2. substances including mechanical contraceptives (condoms, intrauterine devices, tubal rings), disinfectants, and insecticides notified under sub-clause (ii); and
3. devices notified from time to time under sub-clause (iv) of clause (b) of section 3 of the Drugs and Cosmetics Act, 1940;
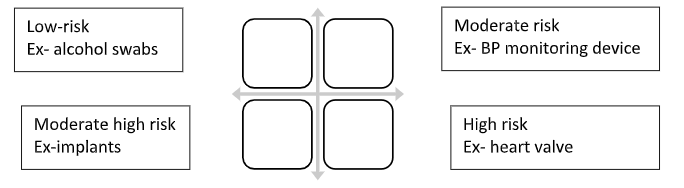
Medical device classification
i) Manufacturing license:
If you are a manufacturer residing in India and want to manufacture medical devices, you must apply for a manufacturing license under CDSCO.

What we do?
RT PRO’s experienced team of consultants can support the manufacturers to get the manufacturing license from CDSCO reasonably and cost-effectively. The list of services provided by RT PRO is as below:
- Guidance on developing the manufacturing site as per the regulatory requirements,
- CDSCO, Zonal and State Drug Controlling Activities application preparation and submission
- Preparation of documentation as per the regulatory requirements
- Providing support in audit preparation and inspection by CDSCO for Class C and Class D medical devices.
- Providing support in audit preparation and inspection by State licensing authority and notified bodies approved by CDSCO for Class A and B medical devices.
Want a free consultation on the Manufacturing license by CDSCO? Contact us. We won’t disappoint you, promise!
ii) Import license:
If you are a manufacturer residing outside India, you need to apply for an import license to sell and distribute the medical device in the Indian market.
What we do?
RT PRO’s experienced team of consultants can support the importers to get the import license from CDSCO reasonably and cost-effectively.
Want a free consultation on the import license by CDSCO? Contact us. We won’t disappoint you, promise!
- Guidance on developing the manufacturing site as per the regulatory requirements,
- CDSCO, Zonal and State Drug Controlling Activities application preparation and submission
- Preparation of documentation as per the regulatory requirements
- Providing support in audit preparation and inspection by CDSCO for Class C and Class D medical devices.
- Providing support in audit preparation and inspection by State licensing authority and notified bodies approved by CDSCO for Class A and B medical devices.
Want a free consultation on the Manufacturing license by CDSCO? Contact us. We won’t disappoint you, promise!